Objective: Provide direction regarding choice of therapy for patients with ulcerative colitis
Patient population: Adult patients (>18 years) with known diagnosis of ulcerative colitis
New therapies are constantly being developed and should be considered.
These clinical decision support tools were developed by Canadian experts in IBD, based on their interpretation of current evidence and considerations specific to Canadian healthcare. International guidelines from Europe and the United States are available. However, these may reflect regional factors not directly applicable in Canada.
Introduction
Ulcerative colitis (UC) is a chronic inflammatory condition of the large intestine limited to the mucosal layer of the colon extending proximally from the rectum, to varying extent. UC is diagnosed based on a combination of clinical presentation, endoscopic findings, and histological features indicating chronic inflammation. It is important to define the extent and severity of inflammation to guide the selection of appropriate treatment and predict prognosis.
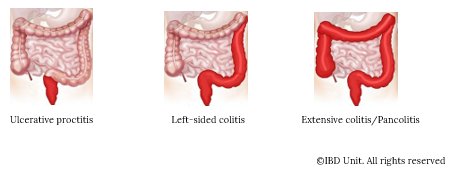
Montreal classification of ulcerative colitis based on disease extent is classified as follows:
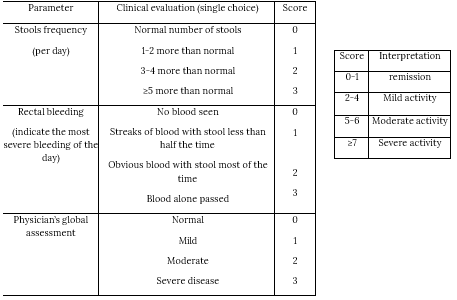
Partial Mayo Scoring System for ulcerative colitis disease activity
Definitions, suggested diagnostic work-up and goal of therapy:
- Corticosteroid refractory UC: If there is no clinical response to oral prednisone (40 to 60 mg or equivalent) within 30 days
- Corticosteroid dependent UC: If corticosteroids cannot be tapered within three months of starting without disease recurrence or if relapse occurs within three months of stopping corticosteroids.
- Laboratory investigation include: CBC, liver biochemical tests, albumin, iron studies, ferritin and CRP
- Stool studies include: Clostridium difficile, routine stool cultures, and fecal calprotectin
- If the patient recently travelled to a parasitic infection endemic region, consider ova and parasites.
- Endoscopy (flexible sigmoidoscopy or colonoscopy) if needed for change in therapy.
- Goal of therapy: To achieve clinical and endoscopic remission.
The following algorithms are best practice clinical pathways for therapy decisions for patients with ulcerative colitis:
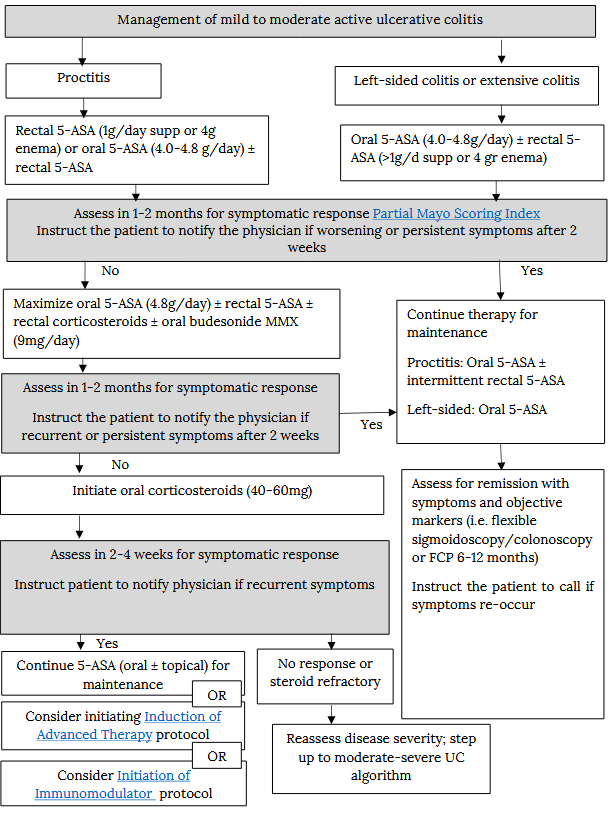
Management of mild to moderate active ulcerative colitis
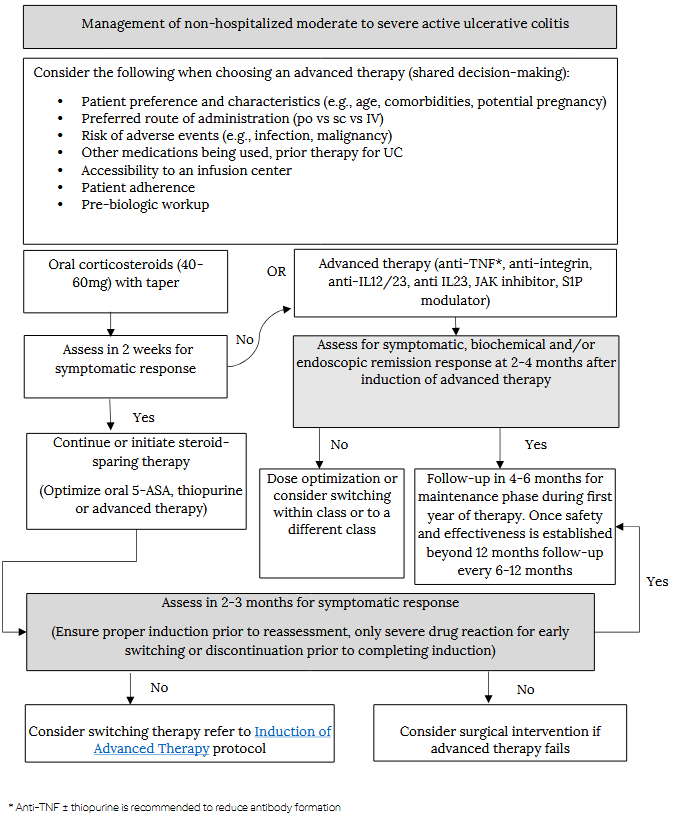
Management of non-hospitalized moderate to severe active ulcerative colitis
Management of hospitalized active severe ulcerative colitis
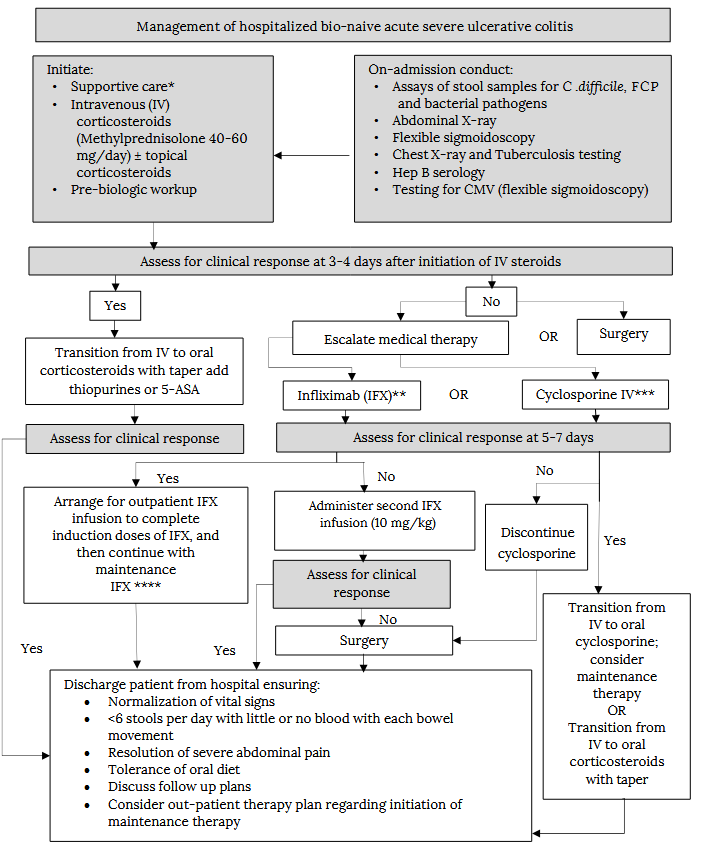
* Monitoring vital signs, stool output, intravenous fluid, electrolyte replacement, venous thromboembolism prophylaxis and nutritional support **The threshold for escalating therapy in time and/or dose should be low, especially in sick patients with low albumin.*** Not commonly used in Canada ****IFX should be administered as combination therapy for at least 6 months on discharge
Note: the role of JAKi in hospitalized patients has not been established and requires expert consultation.
Other Resources
Inflammatory Bowel Disease: Drug Comparison chart
UpToDate® — Patient education: Ulcerative colitis (Beyond the Basics) (freely accessible) https://www.uptodate.com/contents/ulcerative-colitis-beyond-the-basics?topicRef=2004&source=see_link
References
Danese S. et al. Positioning Therapies in Ulcerative Colitis. Clin Gastroenterol and Hepatol 2020; In press
Rubin DT et al. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am J Gastroenterology 2019; 114:384
Bressler and Marshall et al. Clinical Practice Guidelines for the Medical Management of Nonhospitalized Ulcerative Colitis: The Toronto Consensus. Gastroenterology 2015; 148:1035-1058
Bitton A. et al. Treatment of Hospitalized Adult Patients with Severe Ulcerative Colitis: Toronto Consensus Statements. Am J Gastroenterology 2011; 179-194
Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology. 2021;160(5):1570-1583.
